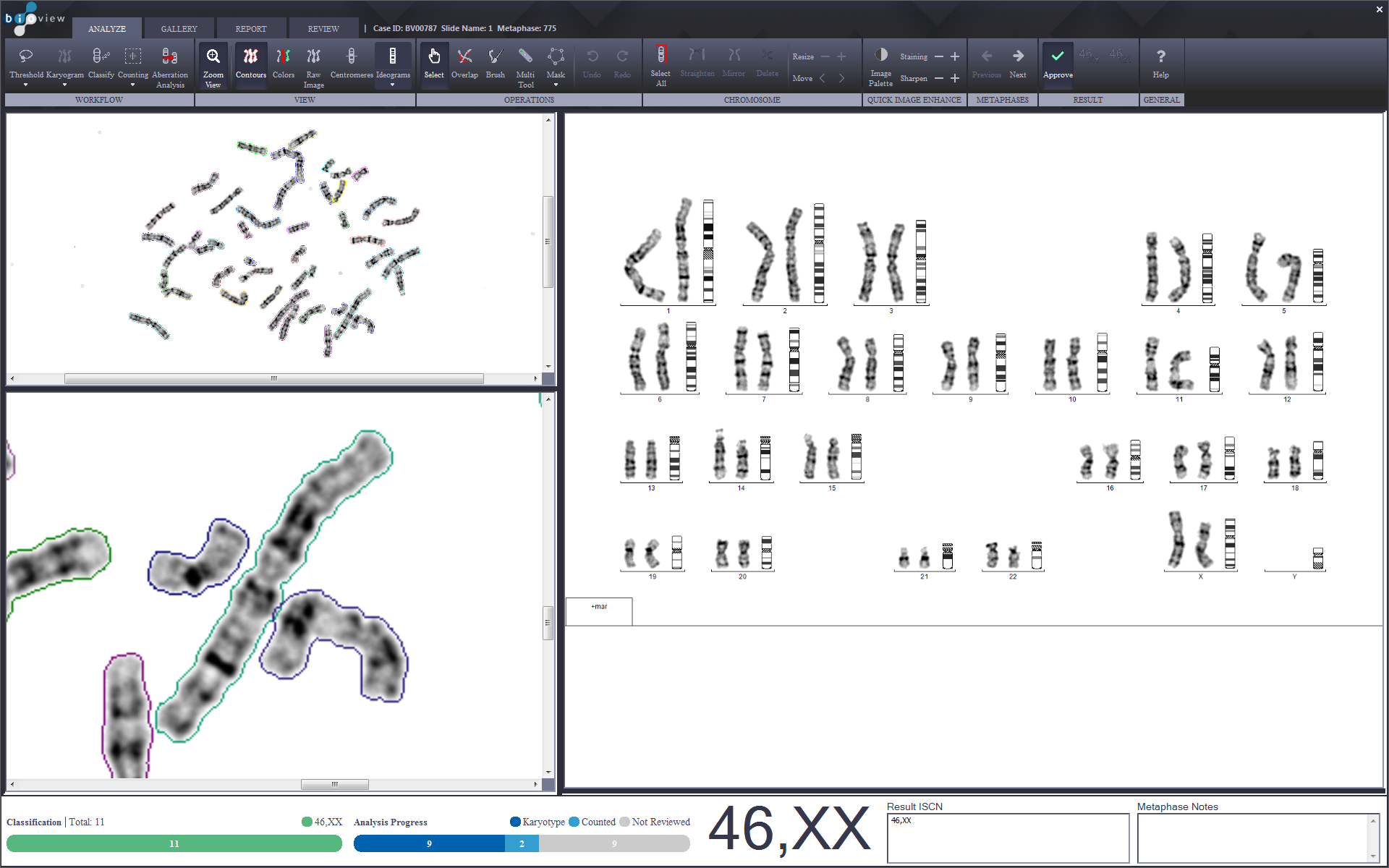
A robust, easy to use integrated solution that streamlines Karyotyping imaging and analysis processes using tools and features professionals require
Revolutionize your lab with BioView’s automated Cytogenetics imaging and analysis platforms.
Introducing streamlined, reliable, and technologically advanced Karyotype and FISH applications that ensure unparalleled precision and ease of use, tailored to your laboratory workflow.
Offering cutting-edge AI-based analysis, innovative features, and tools specifically designed to simplify and expedite case review and reporting. With various solutions fitted to the laboratory
sample volume, desired automation level, and an integrated web application for both local and remote use, BioView’s Cytogenetics Suite is the ideal solution for your laboratory needs.
BioView’s line of imaging platforms ranges from manual capture microscope workstations to various fully automated loader systems. BioView’s solutions can handle different sample types and preparations. Review and analysis can be performed using laboratory computers, dedicated workstations deployed across the laboratory or from any location using standard web browsers. Automated imaging and analysis can be performed for both FISH and Karyotype samples in the same scan batch.
creation BioView’s SoloWeb provides scalable, web-based review, analysis and reporting capabilities, enabling streamlining of the laboratory reporting steps for tissue and cell suspension samples scanned with BioView’s imaging and analysis systems, from any location across the globe via internet access. SoloWeb is designed to optimize the workflow for laboratory technicians, supervisors, and pathologists ,centralizing user management and providing an integrated view of cases status from scan to report. SoloWeb is based on BioView’s experience working with industry leaders, hospitals and laboratories, to provide optimized solutions for connectivity between the lab and the health care provider, as well as to increase productivity and efficiency of the laboratory.
The application is not available in all markets.
For additional information visit our Regulatory Approvals Page